Bactroban Nasal Ointment: Package Insert / Prescribing Info
Package insert / product label
Generic name: mupirocin calcium
Dosage form: intranasal ointment
Drug class: Topical antibiotics
Medically reviewed by Drugs.com. Last updated on Mar 25, 2024.
The Bactroban Nasal brand name has been discontinued in the U.S. If generic versions of this product have been approved by the FDA, there may be generic equivalents available.
See also: Bactroban Cream
On This Page
Bactroban Nasal Ointment Description
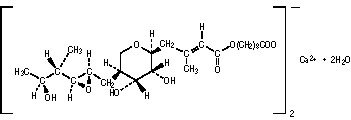
BACTROBAN Nasal (mupirocin calcium ointment, 2%) contains the dihydrate crystalline calcium hemi-salt of the antibiotic mupirocin. Chemically, it is (αE,2S,3R,4R,5S)-5-[(2S,3S,4S,5S)-2,3-Epoxy-5-hydroxy-4-methylhexyl]tetrahydro-3,4-dihydroxy-β-methyl-2H-pyran-2-crotonic acid, ester with 9-hydroxynonanoic acid, calcium salt (2:1), dihydrate.
The molecular formula of mupirocin calcium is (C26H43O9)2Ca•2H2O, and the molecular weight is 1075.3. The molecular weight of mupirocin free acid is 500.6. The structural formula of mupirocin calcium is:
BACTROBAN Nasal is a white to off-white ointment that contains 2.15% w/w mupirocin calcium (equivalent to 2.0% pure mupirocin free acid) in a soft white ointment base. The inactive ingredients are paraffin and a mixture of glycerin esters (SOFTISAN® 649).
Bactroban Nasal Ointment - Clinical Pharmacology
Pharmacokinetics
Following single or repeated intranasal applications of 0.2 gram of BACTROBAN Nasal 3 times daily for 3 days to 5 healthy adult male subjects, no evidence of systemic absorption of mupirocin was demonstrated. The dosage regimen used in this trial was for pharmacokinetic characterization only (see DOSAGE AND ADMINISTRATION for proper clinical dosing information).
In this trial, the concentrations of mupirocin in urine and of monic acid in urine and serum were below the limit of determination of the assay for up to 72 hours after the applications. The lowest levels of determination of the assay used were 50 ng/mL of mupirocin in urine, 75 ng/mL of monic acid in urine, and 10 ng/mL of monic acid in serum. Based on the detectable limit of the urine assay for monic acid, one can extrapolate that a mean of 3.3% (range: 1.2% to 5.1%) of the applied dose could be systemically absorbed from the nasal mucosa of adults.
Data from a report of a pharmacokinetic trial in neonates and premature infants indicate that, unlike in adults, significant systemic absorption occurred following intranasal administration of BACTROBAN Nasal in this population. At this time, the pharmacokinetic properties of mupirocin following intranasal application of BACTROBAN Nasal have not been adequately characterized in neonates or other children younger than 12 years, and in addition, the safety of the product in children younger than 12 years has not been established.
The effect of the concurrent application of intranasal mupirocin calcium ointment, 2% with other intranasal products has not been studied (see PRECAUTIONS, Drug Interactions).
Following intravenous or oral administration, mupirocin is rapidly metabolized. The principal metabolite, monic acid, demonstrates no antibacterial activity. In a trial conducted in 7 healthy adult male subjects, the elimination half-life after intravenous administration of mupirocin was 20 to 40 minutes for mupirocin and 30 to 80 minutes for monic acid. Monic acid is predominantly eliminated by renal excretion. The pharmacokinetics of mupirocin has not been studied in individuals with renal insufficiency.
Microbiology
Mupirocin is an antibacterial agent produced by fermentation using the organism Pseudomonas fluorescens.
Mechanism of Action
Mupirocin inhibits bacterial protein synthesis by reversibly and specifically binding to bacterial isoleucyl transfer‑RNA (tRNA) synthetase.
Mupirocin is bactericidal at concentrations achieved by topical intranasal administration. Mupirocin is highly protein bound (>97%), and the effect of nasal secretions on the minimum inhibitory concentrations (MICs) of intranasally applied mupirocin has not been determined.
Mechanism of Resistance
When mupirocin resistance occurs, it results from the production of a modified isoleucyl-tRNA synthetase, or the acquisition of, by genetic transfer, a plasmid mediating a new isoleucyl-tRNA synthetase. High-level plasmid-mediated resistance (MIC >512 mcg/mL) has been reported in increasing numbers of isolates of Staphylococcus aureus and with higher frequency in coagulase-negative staphylococci. Mupirocin resistance occurs with greater frequency in methicillin-resistant than methicillin‑susceptible staphylococci.
Cross Resistance
Due to its mode of action, mupirocin does not demonstrate cross-resistance with other classes of antimicrobial agents.
Susceptibility Testing
While it is suggested that isolates of S. aureus with a MIC of <256 mcg/mL (absence of high level resistance to mupirocin) may be successfully eliminated from the nares, this criteria should be evaluated at each medical facility in conjunction with laboratory, medical, and infection control staff.1
Correlation of BACTROBAN Nasal in vitro activity and methicillin-resistant Staphylococcus aureus (MRSA) nasal decolonization has been demonstrated in clinical trials (see CLINICAL STUDIES).
Indications and Usage for Bactroban Nasal Ointment
BACTROBAN Nasal is indicated for the eradication of nasal colonization with methicillin-resistant S. aureus in adult patients and healthcare workers as part of a comprehensive infection control program to reduce the risk of infection among patients at high risk of methicillin-resistant S. aureus infection during institutional outbreaks of infections with this pathogen.
NOTE:
- •
- There are insufficient data at this time to establish that this product is safe and effective as part of an intervention program to prevent autoinfection of high-risk patients from their own nasal colonization with S. aureus.
- •
- There are insufficient data at this time to recommend use of BACTROBAN Nasal for general prophylaxis of any infection in any patient population.
- •
- Greater than 90% of subjects/patients in clinical trials had eradication of nasal colonization 2 to 4 days after therapy was completed. Approximately 30% recolonization was reported in 1 domestic trial within 4 weeks after completion of therapy. These eradication rates were clinically and statistically superior to those reported in subjects in the vehicle-treated arms of the adequate and well-controlled trials. Those treated with vehicle had eradication rates of 5% to 30% at 2 to 4 days post-therapy with 85% to 100% recolonization within 4 weeks.
All adequate and well-controlled trials of this product were vehicle-controlled; therefore, no data from direct, head-to-head comparisons with other products are available at this time.
Contraindications
BACTROBAN Nasal is contraindicated in patients with known hypersensitivity to any of the constituents of the product.
Warnings
AVOID CONTACT WITH THE EYES. In case of accidental contact, rinse well with water. Application of BACTROBAN Nasal to the eye under testing conditions has caused severe symptoms such as burning and tearing. These symptoms resolved within days to weeks after discontinuation of the ointment.
In the event of a sensitization or severe local irritation from BACTROBAN Nasal, usage should be discontinued.
Clostridium difficile-associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including BACTROBAN, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C difficile.
C difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing isolates of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial drug use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial drug use not directed against C difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
General
As with other antibacterial products, prolonged use may result in overgrowth of nonsusceptible microorganisms, including fungi (see DOSAGE AND ADMINISTRATION).
Information for Patients
Patients should be given the following instructions:
- •
- Apply approximately one-half of the ointment from the single-use tube directly into 1 nostril and the other half into the other nostril;
- •
- Avoid contact of the medication with the eyes; if BACTROBAN Nasal gets in or near the eyes, rinse thoroughly with water.
- •
- Discard the tube after using, do not re-use;
- •
- Press the sides of the nose together and gently massage after application to spread the ointment throughout the inside of the nostrils; and
- •
- Discontinue usage of the medication and call the healthcare practitioner if sensitization or severe local irritation occurs.
Drug Interactions
The effect of the concurrent application of intranasal mupirocin calcium and other intranasal products has not been studied. Until further information is known, mupirocin calcium ointment, 2% should not be applied concurrently with any other intranasal products.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of mupirocin calcium have not been conducted.
Results of the following studies performed with mupirocin calcium or mupirocin sodium in vitro and in vivo did not indicate a potential for mutagenicity: Rat primary hepatocyte unscheduled DNA synthesis, sediment analysis for DNA strand breaks, Salmonella reversion test (Ames), Escherichia coli mutation assay, metaphase analysis of human lymphocytes, mouse lymphoma assay, and bone marrow micronuclei assay in mice.
Reproduction studies were performed in rats with mupirocin administered subcutaneously at doses up to 40 times the human intranasal dose (approximately 20 mg mupirocin per day) on a mg/m2 basis and revealed no evidence of impaired fertility from mupirocin sodium.
Pregnancy
Teratogenic Effects: Pregnancy Category B
Reproduction studies have been performed in rats and rabbits with mupirocin administered subcutaneously at doses up to 65 and 130 times, respectively, the human intranasal dose (approximately 20 mg mupirocin per day) on a mg/m2 basis and revealed no evidence of harm to the fetus due to mupirocin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Adverse Reactions/Side Effects
Clinical Trials
In clinical trials, 210 domestic and 2,130 foreign adult subjects received BACTROBAN Nasal ointment. Less than 1% of domestic or foreign subjects in clinical trials were withdrawn due to adverse events.
The most frequently reported adverse events in foreign clinical trials were as follows: rhinitis (1.0%), taste perversion (0.8%), pharyngitis (0.5%).
In domestic clinical trials, 17% (36/210) of adults treated with BACTROBAN Nasal ointment reported adverse events thought to be at least possibly drug-related. The incidence of adverse events that were reported in at least 1% of adults enrolled in domestic clinical trials were as follows:
|
Adverse Events |
% of Subjects Experiencing Event BACTROBAN Nasal (n = 210) |
|
Headache |
9% |
|
Rhinitis |
6% |
|
Respiratory disorder, including upper respiratory tract congestion |
5% |
|
Pharyngitis |
4% |
|
Taste perversion |
3% |
|
Burning/stinging |
2% |
|
Cough |
2% |
|
Pruritus |
1% |
The following events thought possibly drug-related were reported in less than 1% of adults enrolled in domestic clinical trials: blepharitis, diarrhea, dry mouth, ear pain, epistaxis, nausea, and rash.
All adequate and well-controlled clinical trials have been performed using BACTROBAN Nasal ointment, 2% in 1 arm and the vehicle ointment in the other arm of the trial. No adequate and well-controlled safety data are available from direct, head-to-head comparative studies of this product and other products for this indication.
Systemic allergic reactions, including anaphylaxis, urticaria, angioedema and generalized rash have been reported in patients treated with formulations of BACTROBAN.
Overdosage
Following single or repeated intranasal applications of BACTROBAN Nasal to adults, no evidence for systemic absorption of mupirocin was obtained. Intravenous infusions of 252 mg, as well as single oral doses of 500 mg of mupirocin, have been well tolerated in healthy adult subjects. There is no information regarding local overdose of BACTROBAN Nasal or regarding oral ingestion of the nasal ointment formulation.
Bactroban Nasal Ointment Dosage and Administration
(See INDICATIONS AND USAGE.)
Adults (aged 12 years and older): Approximately one-half of the ointment from the single-use tube should be applied into 1 nostril and the other half into the other nostril twice daily (morning and evening) for 5 days.
After application, the nostrils should be closed by pressing together and releasing the sides of the nose repetitively for approximately 1 minute. This will spread the ointment throughout the nares.
The single-use 1.0-gram tube will deliver a total of approximately 0.5 grams of the ointment (approximately 0.25 grams/nostril).
The tube should be discarded after usage; it should not be re-used.
The safety and effectiveness of applications of this medication for greater than 5 days have not been established. There are no human clinical or pre-clinical animal data to support the use of this product in a chronic manner or in manners other than those described in this package insert.
Until further information is known, BACTROBAN Nasal should not be applied concurrently with any other intranasal products.
How is Bactroban Nasal Ointment supplied
BACTROBAN Nasal is supplied in 1.0-gram tubes.
NDC 0029-1526-11 (package of 10 single-tube cartons).
Store between 20° and 25°C (68° and 77°F); excursions permitted to 15°-30°C (59°-86°F). Do not refrigerate.
References
- •
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antimicrobial Susceptibility Testing; 22nd Informational Supplement. CLSI Document M100-S22. CLSI, 950 West Valley Rd., Wayne, PA, 19087, 2012.
BACTROBAN and BACTROBAN Nasal are registered trademarks of the GSK group of companies.
SOFTISAN is a trademark of its respective owner and is not a trademark of the GSK group of companies. The maker of this brand is not affiliated with and does not endorse the GSK group of companies or its products.
GlaxoSmithKline
Research Triangle Park, NC 27709
©2014, the GSK group of companies. All rights reserved.
September 2014
BBN:3PI
Principal Display Panel
NDC 0029-1526-11
BACTROBAN NASAL®
(MUPIROCIN CALCIUM OINTMENT 2%)
10 x 1.0 gram Single-Use Tube
Store at 20o - 25oC (68o – 77oF); excursions permitted 15o - 30oC (59o – 86oF).. Do not refrigerate.
Usual Dosage: For intranasal use only. Apply one-half the contents of a tube in one nostril. Apply other half of tube contents in other nostril. See accompanying prescribing information.
GlaxoSmithKline
Research Triangle Park, NC 27709
10000000068393 Rev. 5/09
| BACTROBAN
mupirocin calcium ointment |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - GlaxoSmithKline LLC (167380711) |
Frequently asked questions
- Can I use mupirocin ointment for diaper rash?
- Can mupirocin heal or help with eczema?
- Can mupirocin be used for athletes foot?
- Can mupirocin cream be used for bed sores?
More about Bactroban Nasal (mupirocin topical)
- Compare alternatives
- Side effects
- Dosage information
- During pregnancy
- Drug class: topical antibiotics
- Breastfeeding